Titrate Hcl Against Naoh . between a solution of a strong acid (hcl) and a solution of a strong base (naoh) between a solution of a weak acid (acetic acid. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Repeat titration and boiling till yellow color doesn't return after. since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: titrate with hcl solution till the first color change. Includes kit list and safety. The initial volume and final volume of all three trails were subtracted to find. volume measurements play a key role in titration.
from www.chegg.com
hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. titrate with hcl solution till the first color change. Repeat titration and boiling till yellow color doesn't return after. since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: Includes kit list and safety. between a solution of a strong acid (hcl) and a solution of a strong base (naoh) between a solution of a weak acid (acetic acid. volume measurements play a key role in titration. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The initial volume and final volume of all three trails were subtracted to find.
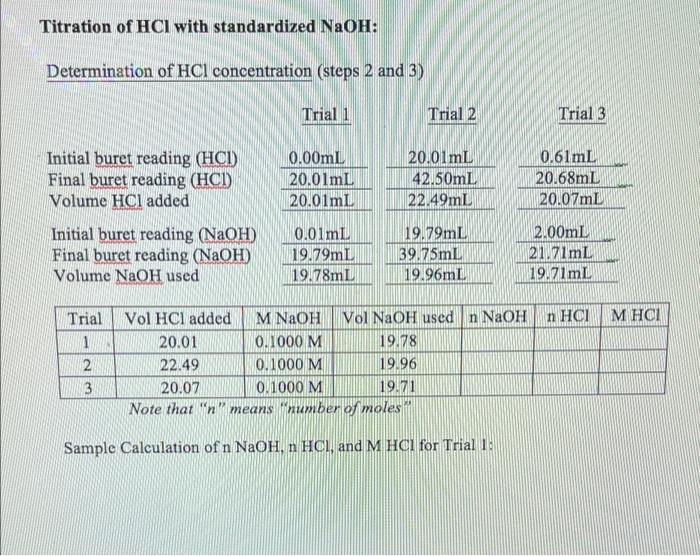
Solved Titration of HCl with standardized NaOH
Titrate Hcl Against Naoh Includes kit list and safety. Repeat titration and boiling till yellow color doesn't return after. The initial volume and final volume of all three trails were subtracted to find. titrate with hcl solution till the first color change. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. between a solution of a strong acid (hcl) and a solution of a strong base (naoh) between a solution of a weak acid (acetic acid. volume measurements play a key role in titration. since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Includes kit list and safety.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Titrate Hcl Against Naoh hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. between a solution of a strong acid (hcl) and a solution of a strong base (naoh) between a solution of a weak acid (acetic acid. The initial volume and final volume of all three trails were. Titrate Hcl Against Naoh.
From mungfali.com
HCl NaOH Titration Titrate Hcl Against Naoh hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. volume measurements play a key role in titration. titrate with hcl solution till the first color change. The initial volume and final volume of all three trails were subtracted to find. since hcl and. Titrate Hcl Against Naoh.
From exotnsiwz.blob.core.windows.net
Titration Of Naoh With Hcl at Katherine Grassi blog Titrate Hcl Against Naoh since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. between a solution of a strong acid (hcl) and a solution of a strong. Titrate Hcl Against Naoh.
From exotnsiwz.blob.core.windows.net
Titration Of Naoh With Hcl at Katherine Grassi blog Titrate Hcl Against Naoh hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. volume measurements play a key role in titration. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety. between a solution. Titrate Hcl Against Naoh.
From byjus.com
The graph of pH during the titration of NaOH and HCl Titrate Hcl Against Naoh hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. volume measurements play a key role in titration. Repeat titration and boiling till yellow color doesn't return after. The initial volume and final volume of all three trails were subtracted to find. between a solution. Titrate Hcl Against Naoh.
From dxopcuxtp.blob.core.windows.net
Titration Experiment Hydrochloric Acid And Sodium Hydroxide at Silvia Titrate Hcl Against Naoh Includes kit list and safety. The initial volume and final volume of all three trails were subtracted to find. since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: between a solution of a strong acid (hcl) and a solution of a strong base (naoh) between a. Titrate Hcl Against Naoh.
From dokumen.tips
(PDF) AcidBase Titration NaOH with HCL DOKUMEN.TIPS Titrate Hcl Against Naoh use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The initial volume and final volume of all three trails were subtracted to find. titrate with hcl solution till the first color change. hcl and naoh are strong acid and strong base respectively and their titration curves are similar. Titrate Hcl Against Naoh.
From dxoqraial.blob.core.windows.net
Titration Reaction Of Hcl And Naoh at Lucia Chamberlain blog Titrate Hcl Against Naoh Repeat titration and boiling till yellow color doesn't return after. since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: between a solution of a strong acid (hcl) and a solution of a strong base (naoh) between a solution of a weak acid (acetic acid. volume. Titrate Hcl Against Naoh.
From www.studocu.com
Titration Report Titrating Acetic Acid and NaOH AcidBase Titration Titrate Hcl Against Naoh Includes kit list and safety. titrate with hcl solution till the first color change. volume measurements play a key role in titration. since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: between a solution of a strong acid (hcl) and a solution of a. Titrate Hcl Against Naoh.
From exoptndih.blob.core.windows.net
Acid Base Titration Lab Answers Hcl Naoh at Daniel Tilley blog Titrate Hcl Against Naoh between a solution of a strong acid (hcl) and a solution of a strong base (naoh) between a solution of a weak acid (acetic acid. volume measurements play a key role in titration. titrate with hcl solution till the first color change. hcl and naoh are strong acid and strong base respectively and their titration curves. Titrate Hcl Against Naoh.
From www.researchgate.net
Titration of HCl (0.1M) against NaOH (0.1M) Download Scientific Diagram Titrate Hcl Against Naoh hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Repeat titration and boiling till yellow color doesn't return after. The initial volume and final volume of all three trails were subtracted to find. use this class practical to explore titration, producing the salt sodium chloride. Titrate Hcl Against Naoh.
From mariela-kcarroll.blogspot.com
Titration Curve of Hcl and Naoh Titrate Hcl Against Naoh hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety. The initial volume and final volume of all three trails were subtracted to. Titrate Hcl Against Naoh.
From www.sarthaks.com
conductometric titration curve of a equimolar mixture of a HCl and HCN Titrate Hcl Against Naoh use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Repeat titration and boiling till yellow color doesn't return after. between a solution of a strong acid (hcl) and a solution of a strong base (naoh) between a solution of a weak acid (acetic acid. hcl and naoh are. Titrate Hcl Against Naoh.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Titrate Hcl Against Naoh between a solution of a strong acid (hcl) and a solution of a strong base (naoh) between a solution of a weak acid (acetic acid. The initial volume and final volume of all three trails were subtracted to find. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. . Titrate Hcl Against Naoh.
From www.doubtnut.com
Which of the following plot represents the graph of pH against volume Titrate Hcl Against Naoh The initial volume and final volume of all three trails were subtracted to find. Includes kit list and safety. volume measurements play a key role in titration. since hcl and naoh fully dissociate into their ion components, along with sodium chloride (nacl), we can rewrite the equation as: hcl and naoh are strong acid and strong base. Titrate Hcl Against Naoh.
From www.chegg.com
Solved Titration of HCl with standardized NaOH Titrate Hcl Against Naoh use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety. The initial volume and final volume of all three trails were subtracted to find. Repeat titration and boiling till yellow color doesn't return after. since hcl and naoh fully dissociate into their ion components, along. Titrate Hcl Against Naoh.
From www.studypool.com
SOLUTION Titration of hydrochloric acid against sodium hydroxide Titrate Hcl Against Naoh Includes kit list and safety. between a solution of a strong acid (hcl) and a solution of a strong base (naoh) between a solution of a weak acid (acetic acid. The initial volume and final volume of all three trails were subtracted to find. hcl and naoh are strong acid and strong base respectively and their titration curves. Titrate Hcl Against Naoh.
From www.youtube.com
Conductometric titration I strong acid (HCl) versus strong base Titrate Hcl Against Naoh use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety. hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. The initial volume and final volume of all three trails were subtracted to. Titrate Hcl Against Naoh.